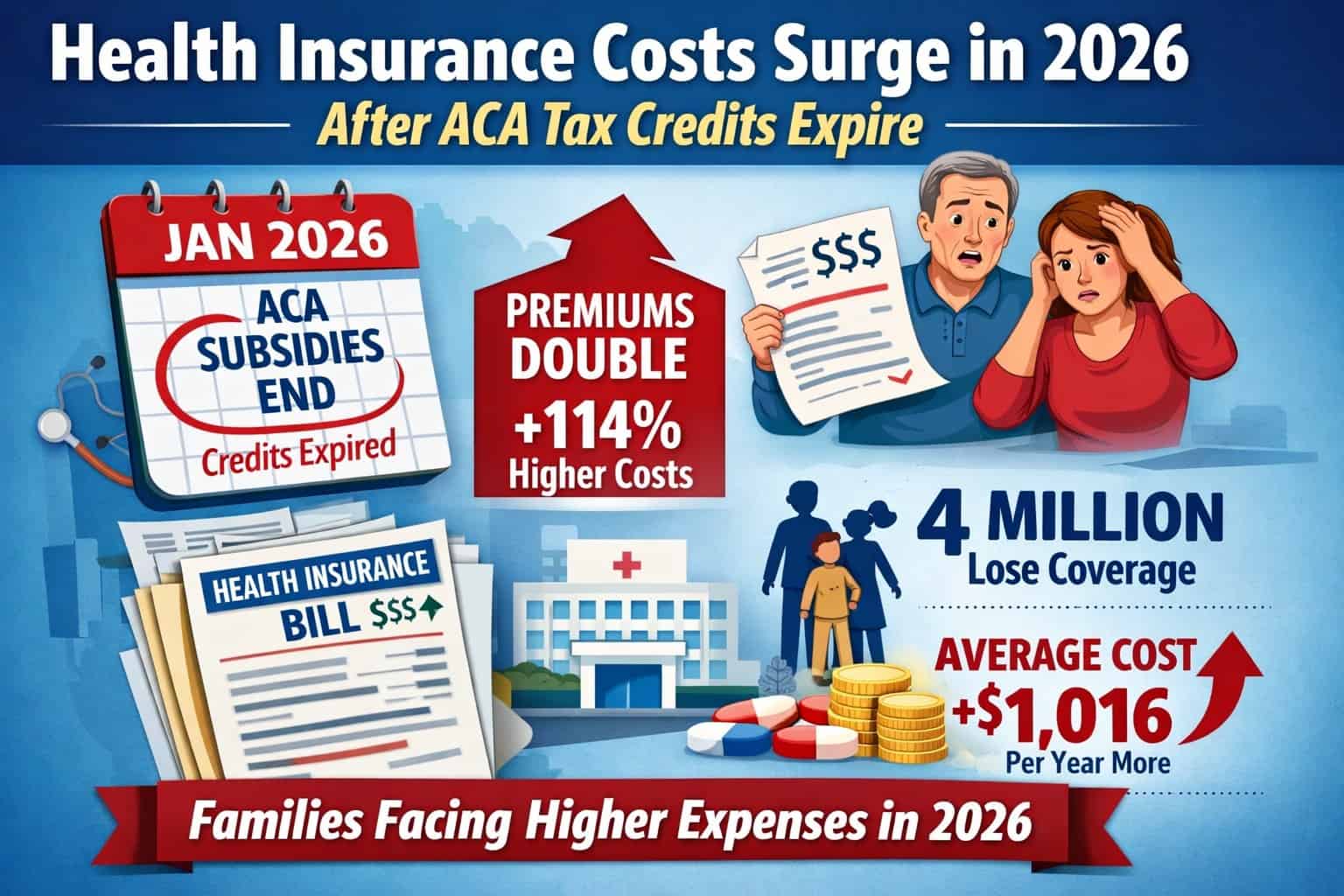
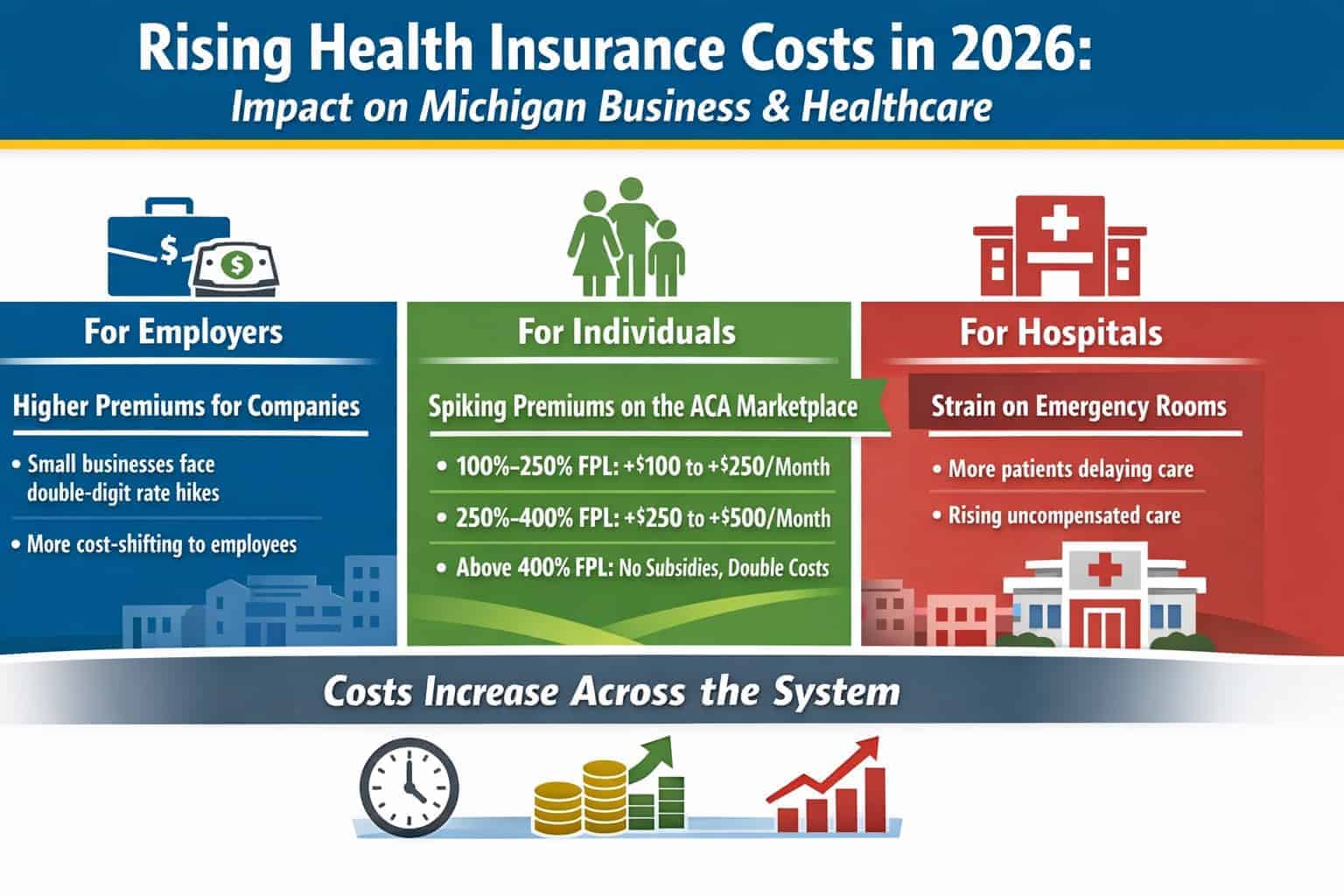
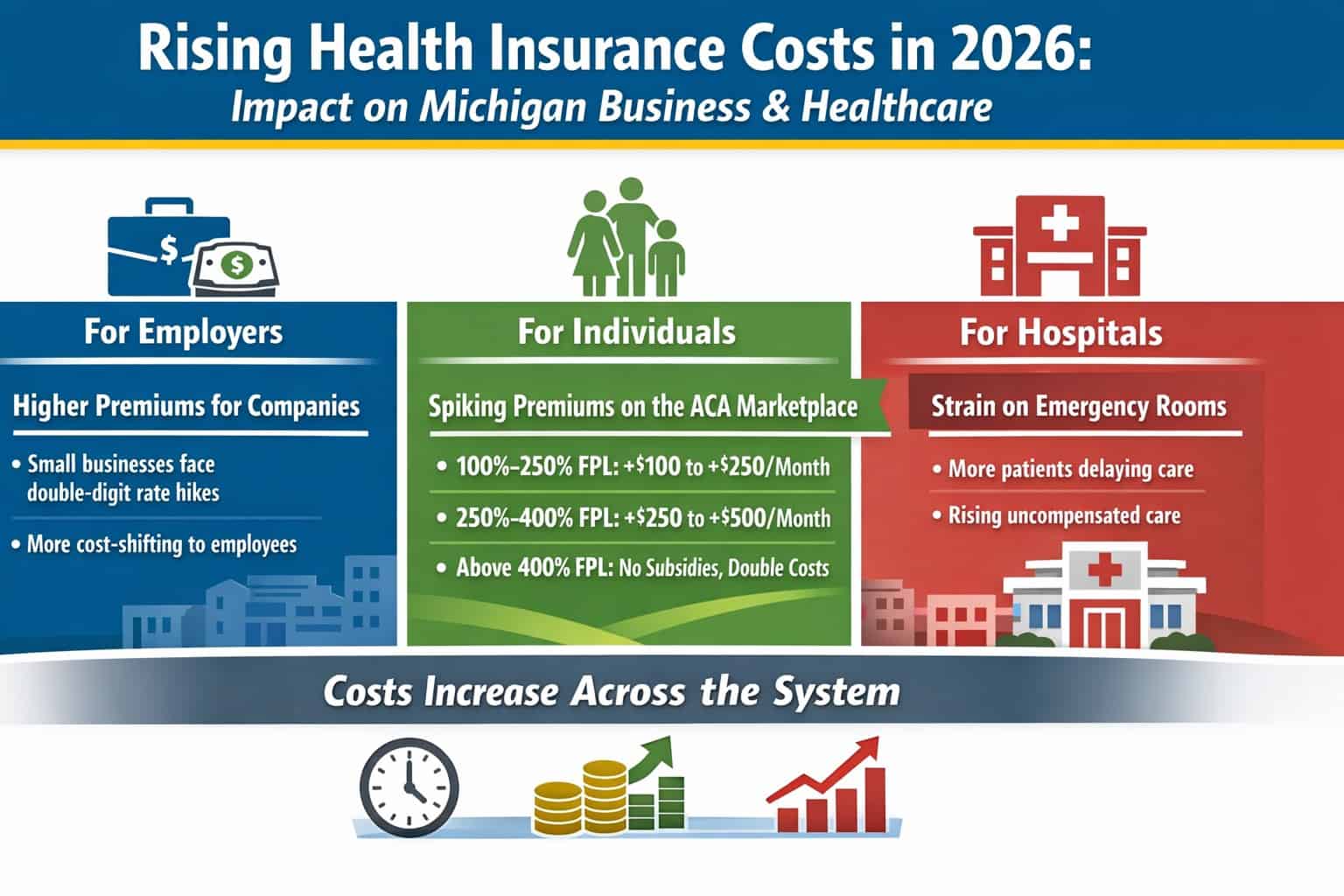
ANN ARBOR – MichBio members can get an update on the latest legislative and regulatory issues facing the medical device and diagnostic sectors when the life sciences trade association President and CEO Stephen Rapundalo hosts the online program prior to his Capitol Hill visit, as part of the AdvaMed Legislative Fly-In set for February 26-27.
The medical device tax, tax reform, regulatory compliance, coverage and reimbursement, patent reform and IP protection are just a few of the topics that will be discussed.
Join us and make sure that you’re current on matters that can impact your operations and bottom line. We need your input to best represent your interests!
Register for the Capitol Update webinar by clicking on GoToMeeting.Com
FDA Finalizes Rule On E-Reporting Of Device-Related Adverse Events
The FDA has released a final rule requiring medical device companies to report in an electronic format any adverse events linked to their products – also known as medical device reports (MDRs). The electronic reporting process is meant to streamline the way the FDA evaluates adverse events so that it can promptly alert the industry, other federal agencies, providers and patients about potential threats, the rule states. A FAQ page is available on the FDA Center for Devices & Radiologic Health (CDRH) website regarding the final guidance governing MDRs.
Guidance Issued on Requests for Feedback on Medical Device Submissions
The FDA CDRH has released a guidance that provides an overview of the mechanisms available to applicants through which they can request feedback from the FDA regarding potential or planned medical device Investigational Device Exemption (IDE) applications or other premarket submissions, such as Premarket Approval (PMA) applications, Humanitarian Device Exemption (HDE) applications, Evaluation of Automatic Class III Designations (de novo petitions), Premarket Notification (510(k)) Submissions, Clinical Laboratory Improvement Amendments (CLIA) Waiver by Application, and including certain Investigational New Drug Applications (INDs) and Biologics License Applications (BLAs).
This guidance provides information regarding the logistics for submission, receipt, tracking, and review of/response to these requests. To view the guidance, Click on FDA.Gov
On February 26, the FDA will hold a webinar to help explain the guidance and to provide a forum for asking questions you may have regarding this guidance. Registration is not necessary.
Webinar Details:
Time: 2:30 – 4:00 PM, Eastern Time
To ensure you are connected, please connect at 2:15 PM.
To hear the presentation and ask questions: Dial: 800-857-9818, passcode: CDRH