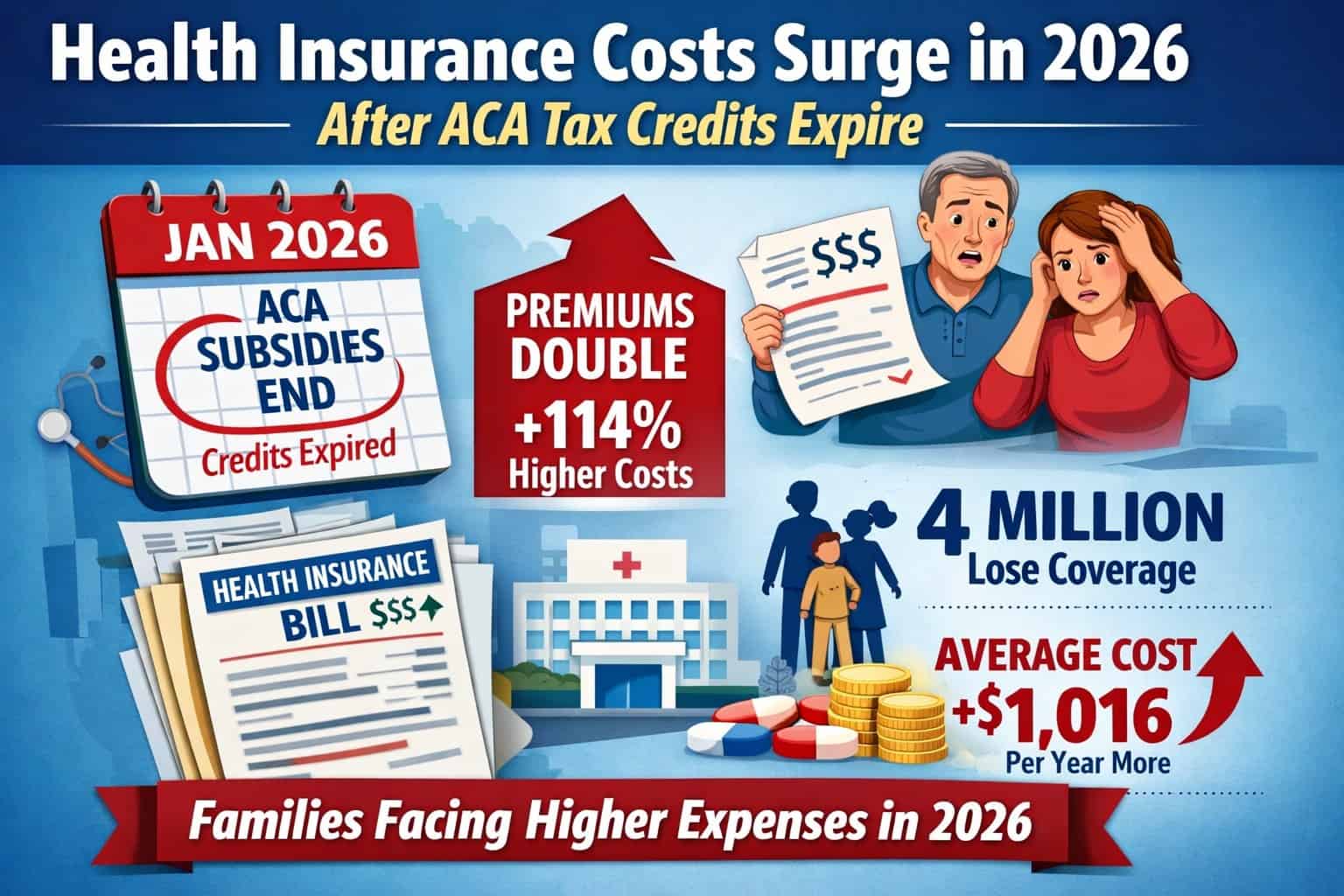
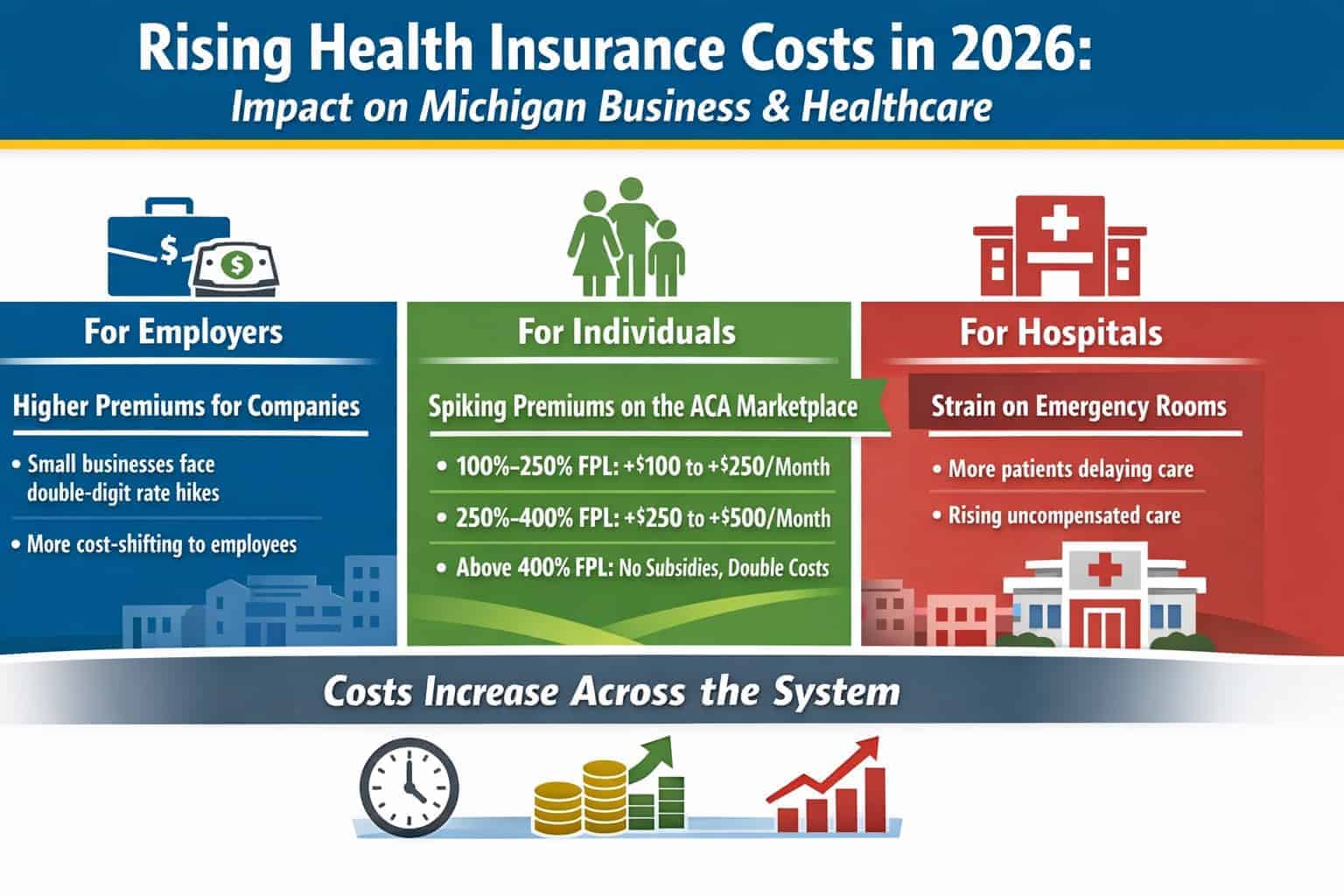
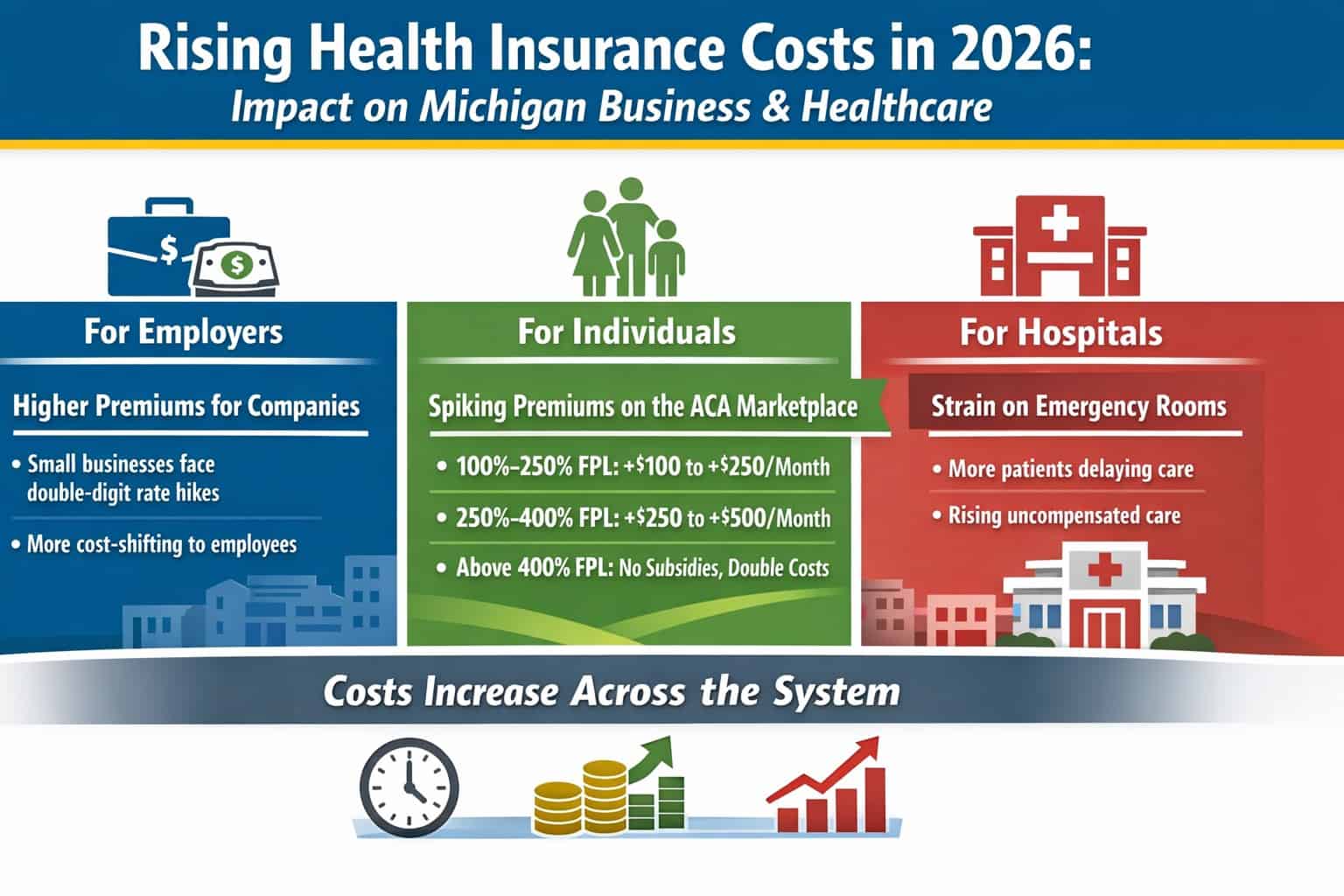
ANN ARBOR – ONL Therapeutics, a biopharmaceutical company developing novel therapies for preserving sight in a range of retinal diseases, Wednesday announced it has been awarded a $1.37 million Small Business Innovation Research Phase II contract from the National Eye Institute to advance development of ONL101, the company?s lead therapeutic candidate.
ONL101 is a first-in-class small molecule peptide initially being developed for the protection of photoreceptors in retinal detachment, a condition for which the product has been granted orphan disease designation. This grant will be used to support the remaining ONL101 preclinical development activities required for submission of an investigational new drug (IND) application.
ONL received this funding following the successful completion of a Phase I SBIR project which focused on demonstrating the feasibility of the company?s approach to blocking photoreceptor apoptosis in retinal detachment animal models. This NEI-funded research showed that ONL could deliver the target molecule intravitreally and achieve sufficient retinal concentrations to block apoptosis.
?Receipt of this second SBIR grant from the National Eye Institute provides further validation for our foundational photoreceptor protection technology, as well as our ONL101 product,? said John Freshley, chief executive officer of ONL Therapeutics. ?This funding will allow for continued evaluation of ONL101?s ability to protect photoreceptors in cases of retinal detachment and, ultimately, preserve vision. Illustrating this mechanism of photoreceptor protection not only helps advance ONL101 as a treatment for retinal detachment but also provides rationale for the technology?s application to other important retinal diseases such as wet and dry age-related macular degeneration.?
In addition to the SBIR grant, ONL has also raised $1 million through a convertible bridge loan from a syndicate led by the Capital Community Angels of Lansing, Michigan. Additional investors included Biosciences Research & Commercialization Center (with funds from the Michigan Economic Development Corporation), ONL management and other unnamed investors. The bridge loan will convert to Series A Preferred shares concurrent with the closing of a Series A financing. Combined, the SBIR grant and bridge loan provide ONL with sufficient capital to complete IND-enabling studies for ONL101.
ONL is currently preparing for IND-enabling preclinical activities for ONL101 with the expectation of submitting an IND application in late 2015 to support the initiation of a Phase 1 clinical trial in retinal detachment. The company has already completed a pre-IND meeting with the United States Food and Drug Administration (FDA) and the agency has deemed the company?s clinical development plan for ONL101 as acceptable.