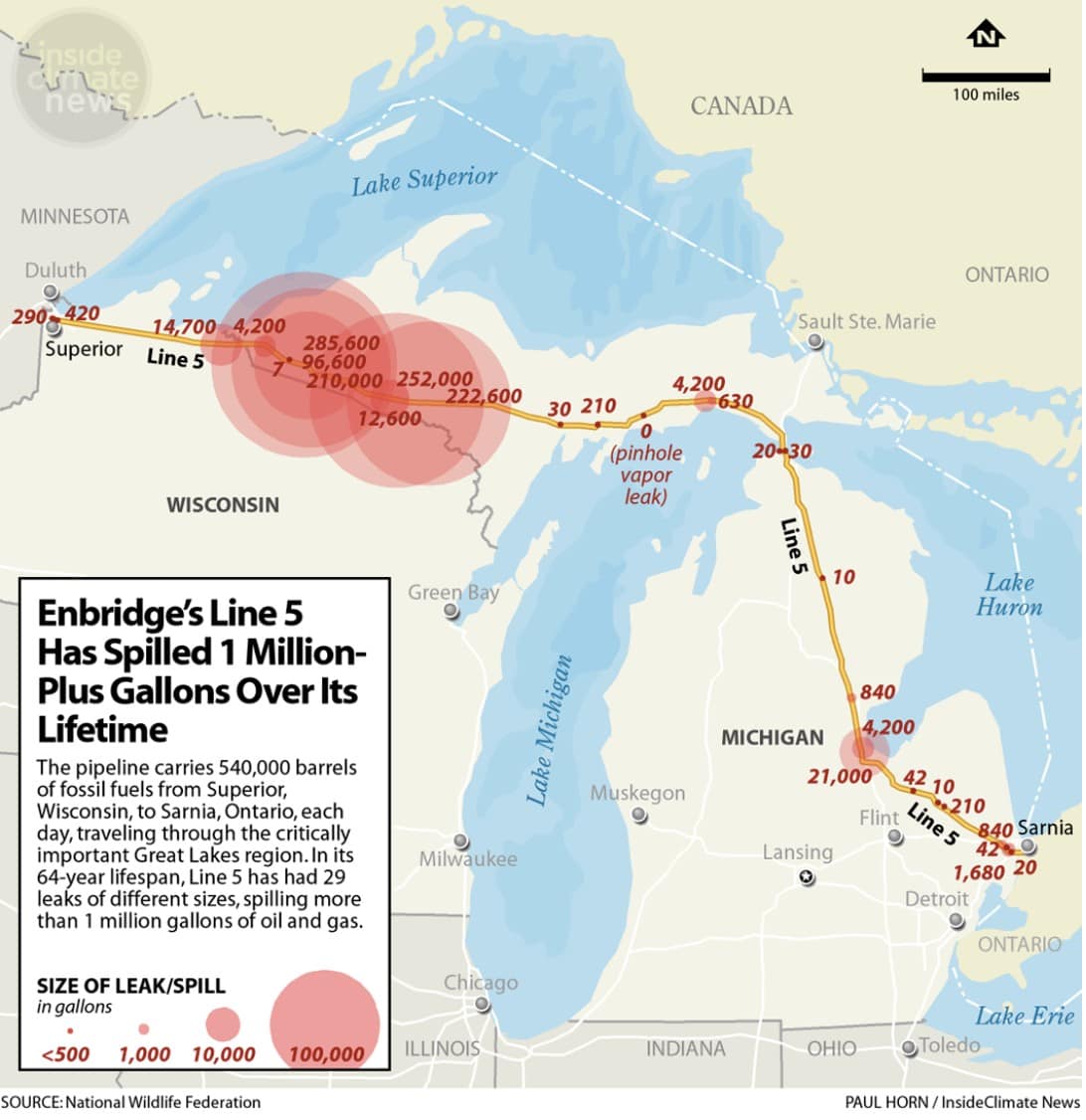
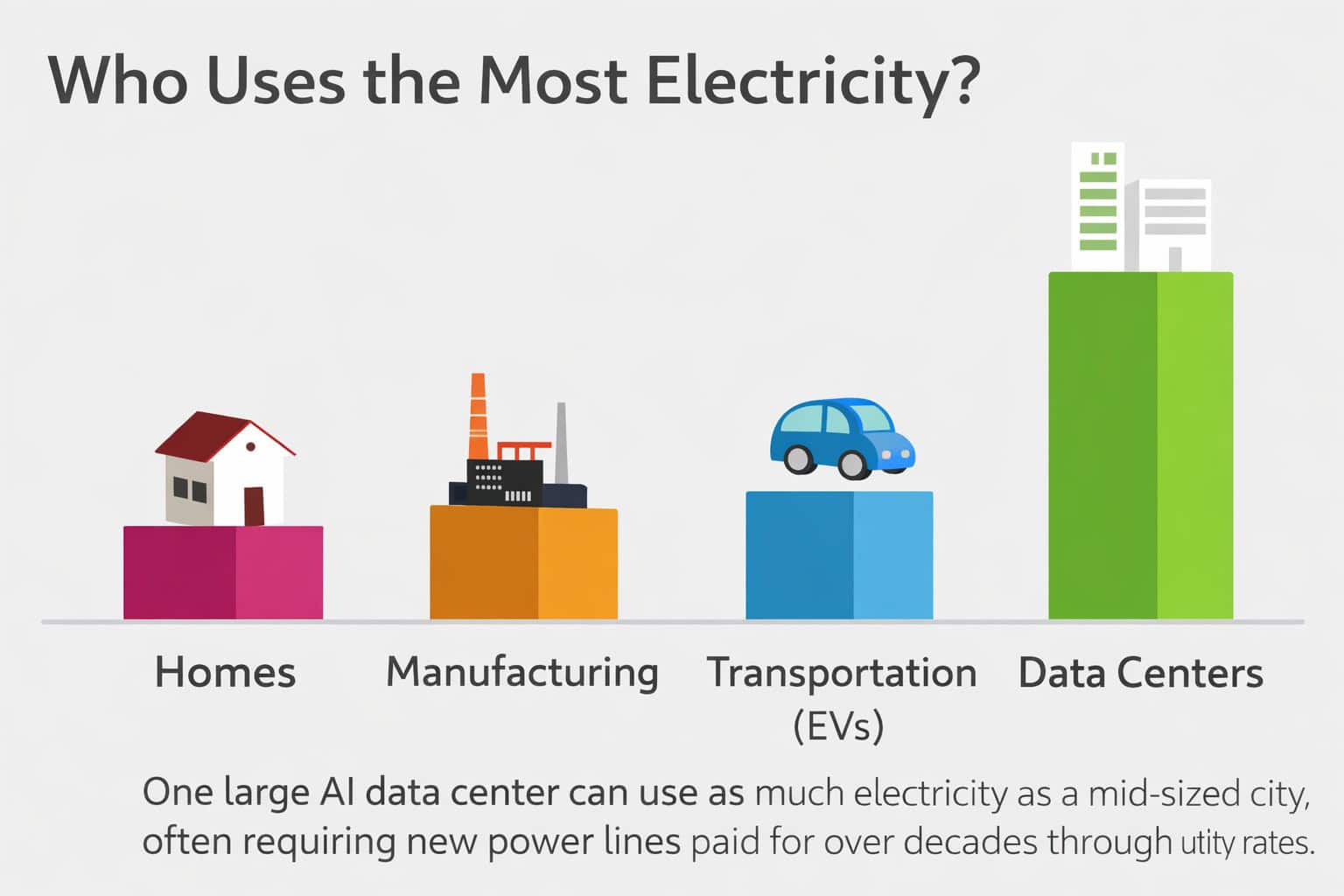
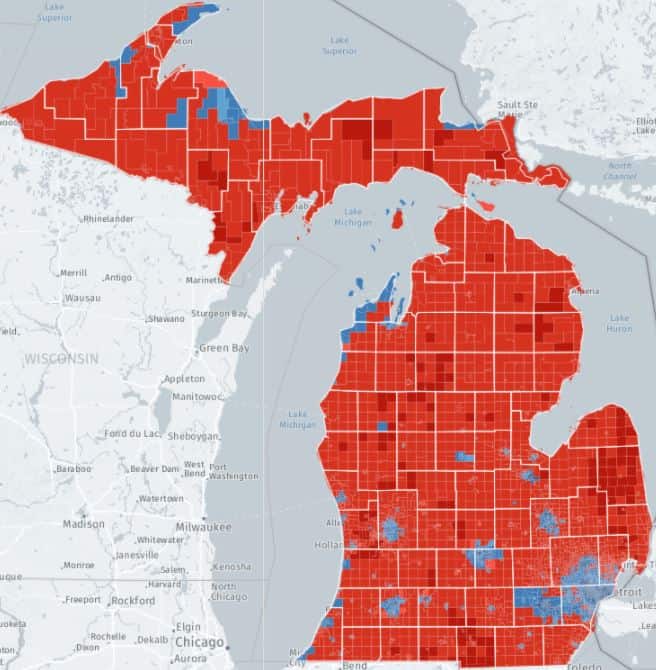
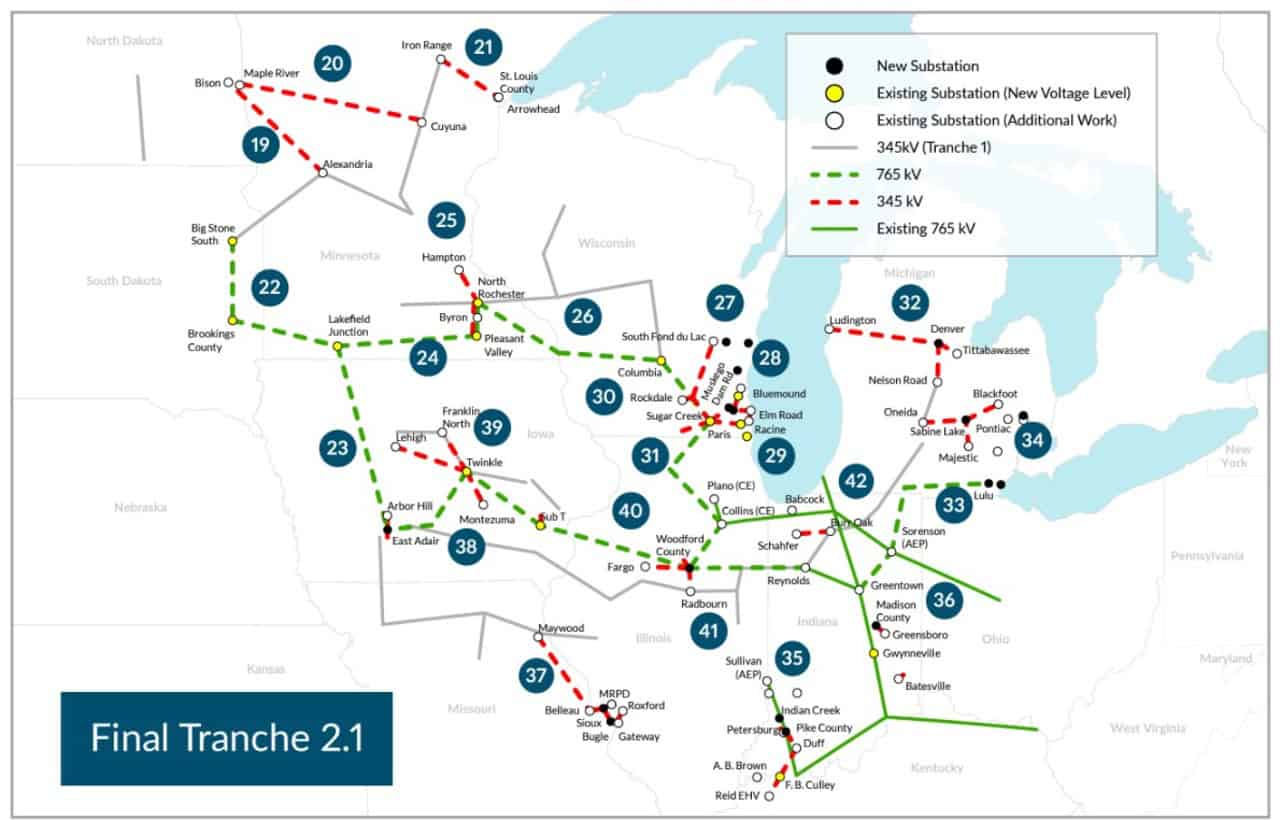
FLINT – With support from the Michigan Soybean Promotion
Committee, Jonathan Wenzel, assistant professor of Chemical Engineering at
Kettering University, is attempting to speed up the process of making biodiesel
– a renewable fuel that is made using predominantly soybean oil in the United
States, but can be made from animal fats and other vegetable oils.
Wenzel’s scientific mission: transform the process of making
biodiesel by increasing the speed at which soybean oil reacts with methanol to
produce biodiesel.
Methanol and soybean oil react to create biodiesel. However,
methanol and soybean oil do not amalgamate readily and require heat, vigorous
mixing, a catalyst and lots of time to react. The methodology Wenzel is using,
a process that has been under study at other universities, provides improved
results.
“Our approach was to take methanol, heat it and pressurize
it beyond its critical point to create supercritical methanol,” Wenzel said. “Supercritical methanol can more readily dissolve the soybean oil, and combined
with the higher temperatures we can react it without a catalyst.”
Supercritical methanol is created by elevating the
temperature and pressure of regular methanol to the critical point that
encourages it to act as a liquid and gas simultaneously which gives it unique
solvent properties. Using this methodology, Wenzel asserts that
biodiesel, which can be used to fuel engines that run on diesel, can be made in
under 10 minutes. The methodology, when increased in scale, may serve as an
economic opportunity.
“There are no supercritical methanol biodiesel plants in the
United States,” Wenzel said. “With the data we produced, we had chemical
engineering students conduct plant simulations to see if this was economically
viable. The answer is yes, you can make a profit by producing biodiesel using
this methodology.”
Kettering student Jason Davis presented parts of these
findings at the Michigan Academy of Science, Arts, and Letters in March 2015 at
Andrews University in Berrien Springs, Michigan. Wenzel also presented the
findings at the American Institute of Chemical Engineers conference on November
19, 2014 in Atlanta, Georgia.
Wenzel is grateful to the Michigan Soybean Promotion
Committee for funding the first phase of this research. The next step is to
improve the process from the current structure of making the biodiesel in
batches, which is labor intensive, to a more automated flow system. After the
process is tested, the following step is to scale up from a small reactor to a
larger one and then possibly a pilot biodiesel plant to see if the fuel can be manufactured
on a commercial scale.
An influx in demand for biodiesel would create a market for
soybean oil but also waste grease and other fatty products which can all be
mixed with methanol to produce biodiesel. The demand for biodiesel and waste
substrates required to produce it will depend on the potential applications of
the renewable fuel. At present the United States produces a billion
gallons of biodiesel a year.
“Several municipalities run their buses on it,” Wenzel said. “You can buy biodiesel and blended biodiesel for cars.”
Kettering University is a national leader in experiential
STEM (science, technology, engineering and math) education, integrating an
intense academic curriculum with applied professional experience. Through this
proven approach we inspire students to realize their potential and advance
their ideas by combining theory and practice better than any institution in the
world. Kettering University is dedicated to achieving the extraordinary through
technological innovation, leadership and service, built on values that foster
respect, integrity, creativity, collaboration and excellence in growth, global
leadership, community outreach and an engaged community of stakeholders.